FREE SHIPPING OVER $600 MINIMUM ORDER $400
NEURAMIS DEEP
$33.00
62 in stock
Minimum order $400
Free shipping $600
- Add to cart Products
- Login
- Place order
- Pay order Online
- Receive the Products
Description
Neuramis Deep | Cross-Linked Hyaluronic Acid Filler for Deep Wrinkles
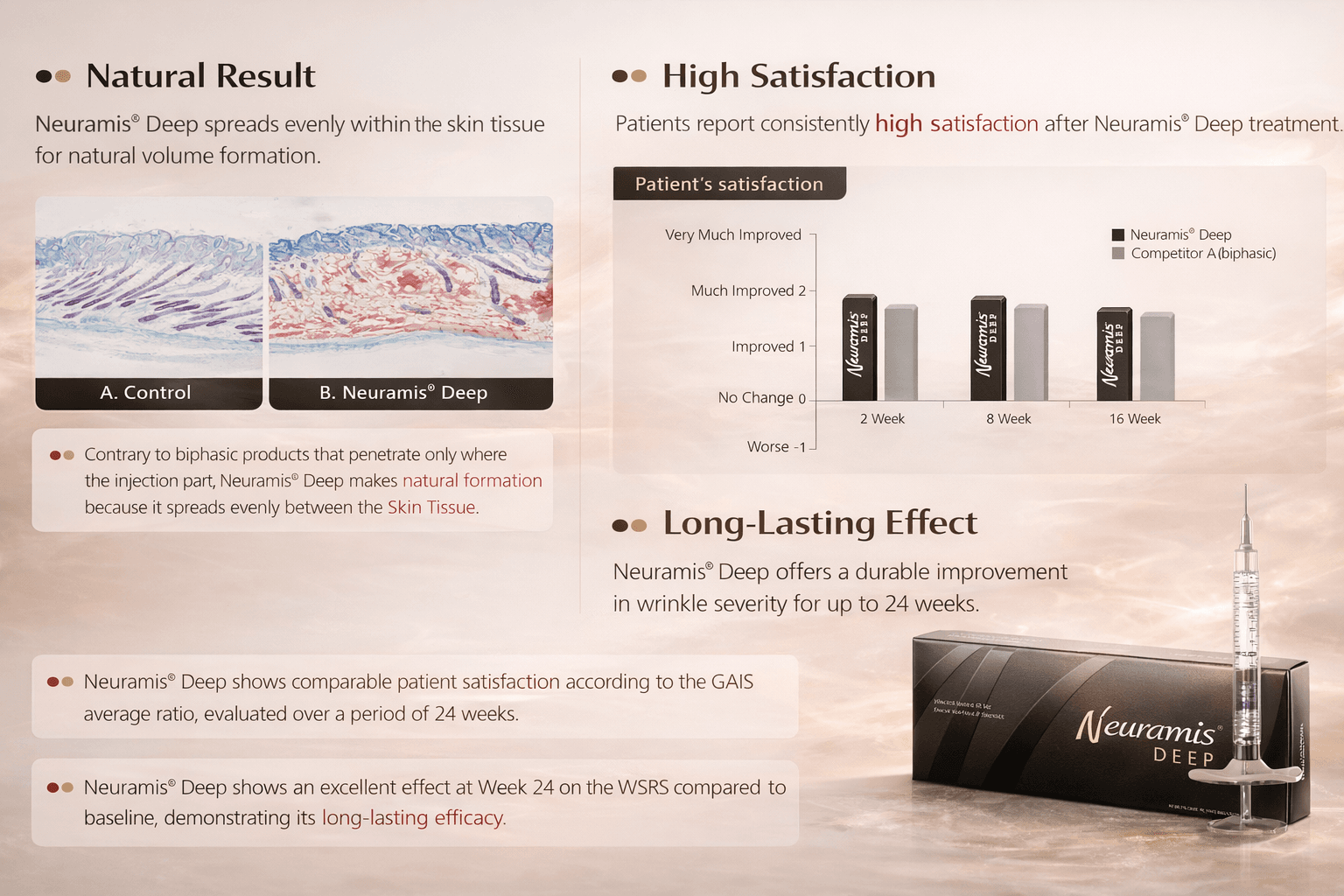
Neuramis Deep is a cross-linked hyaluronic acid (HA) dermal filler designed for correction of moderate-to-deep, static facial wrinkles and for facial contouring/volume augmentation.
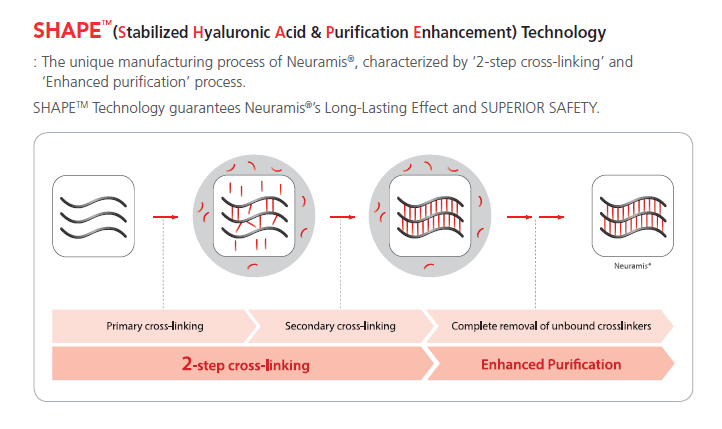
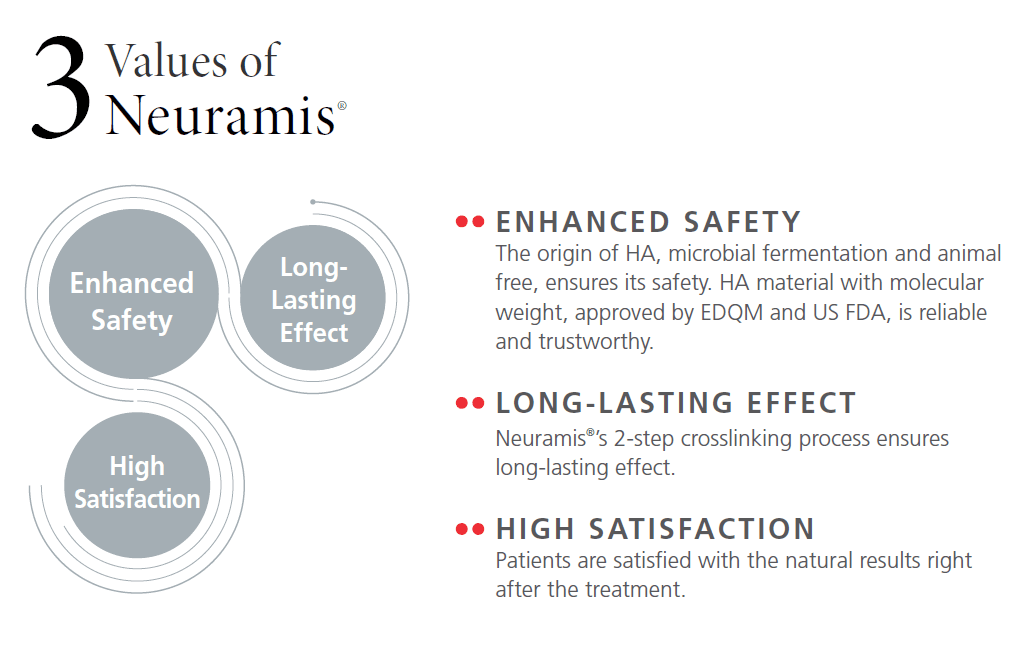
It is part of the Neuramis® range produced by Medytox and manufactured using the company’s SHAPE™ process (2-step cross-linking plus enhanced purification).
Neuramis® is positioned for skin rejuvenation use cases where HA gel is used to support volume and reduce the visible depth of folds.
Hyaluronic acid is a naturally occurring polymer found in living organisms and is present in human skin. In dermal filler products, HA is used in a cross-linked gel form to improve stability and persistence compared with non-cross-linked HA.
Key Features
- HA concentration: 20 mg/mL (Neuramis Deep)
- Gel type: Cross-linked HA gel (stabilized)
- Manufacturing process: SHAPE™ technology (two-step cross-linking + enhanced purification)
- Purification focus: Minimization of residual (uncross-linked) BDDE (as stated in manufacturer/competitor content)
- Needle reference (commonly listed): 27G × 1/2” (13 mm)
Function: How Neuramis Deep Works
Hyaluronic acid is a naturally occurring polymer found in living organisms and in human skin. In the skin, it contributes to hydration through its ability to bind water. In dermal filler formulations, hyaluronic acid is cross-linked to improve stability compared with non-cross-linked HA, allowing the gel to maintain form after placement.
Cross-linked HA gels interact with naturally present hyaluronic acid in the skin and are commonly used to provide temporary volume support. The duration of this support varies and is influenced by factors such as skin characteristics, wrinkle depth, injection technique, and the amount of product used.
Neuramis Deep contains a stabilized hyaluronic acid gel intended to support volume and structure in areas with visible lines, folds, or contour changes.
SHAPE™ technology describes a manufacturing process that combines two-step cross-linking with enhanced purification. The two-step cross-linking process increases gel stability, while the purification stage is designed to reduce residual uncross-linked BDDE in the final formulation.
Because hyaluronic acid binds water, HA-based gels are commonly associated with changes in surface hydration and the visible appearance of skin texture following treatment. These effects depend on the treatment area and the technique applied by a licensed medical professional.
Benefits Of Neuramis Deep: Hydration, Texture, Glow
Results depend on individual factors and clinician technique.
- Skin hydration appearance: HA’s water-binding properties are commonly used to support a more hydrated-looking surface.
- Texture appearance: By addressing volume loss and deeper folds, treated areas may appear smoother in still facial positions.
- Glow and overall look: When volume deficiencies and deeper lines are reduced, the face may look more refreshed due to improved contour continuity (an appearance effect rather than a performance guarantee).
Indication For Use (What It Is Commonly Used For)
Neuramis Deep is indicated for deep wrinkles and facial contouring. Facial contouring/augmentation areas that are frequently referenced for HA fillers, including
- Moderate-to-deep folds such as nasolabial folds and other facial wrinkles.
- Forehead lines/forehead augmentation areas
- Cheeks and cheek contouring
- Chin and jawline contouring
- Lip volume correction (where listed for Deep/Deep Lidocaine)
- Nose contouring (where listed)
- Marionette lines (where listed)
Important use note (non-medical guidance): Dermal filler injections should be performed only by trained, licensed medical professionals.
Main Ingredients
Neuramis Deep (CE1293)
- Cross-linked Hyaluronic Acid (HA), 20 mg/mL: Provides a stabilized gel structure used for volume support and the appearance of wrinkle reduction and facial contouring.
Neuramis Deep Lidocaine
- Cross-linked Hyaluronic Acid (HA), 20 mg/mL: Same base gel concentration referenced for the Deep range.
- Lidocaine, 0.3% (w/v): Included in the “with lidocaine” variant to reduce injection discomfort (as described in competitor content).
Neuramis Deep Application Technique
Because outcomes and safety depend heavily on placement depth, anatomy, and technique, practitioners commonly use established injection approaches selected per facial area and indication.
The following are commonly referenced techniques in professional filler practice (overview only):
- Tunneling/linear threading: Filler is placed along a line to address wrinkles or folds, often aiming for even distribution along the target.
- Point technique (serial puncture): Multiple small deposits are placed in close proximity to build correction across a region.
- Stretching technique: Manual tension on surrounding skin can improve control and visibility during placement in some areas.
- Dual-plane technique: Product may be placed at two distinct tissue levels in the same region when a practitioner determines layered support is appropriate.
These technique labels are general conventions. Selection and execution are clinician-dependent and should follow local regulations, training standards, and product instructions.
Packing Information (Size, Unit, Quantity, Format)
Package formats vary across the provided market listings. Common configurations referenced include:
- Pack size: 1 syringe (1.0–1.1 mL, market-dependent)
- Needle: 27G × 1/2” (13 mm) (commonly listed)
Manufacturer Details
- Manufacturer: Medytox Inc., Ltd.
- Country of manufacture referenced: South Korea
- Brand line: Neuramis® (full range includes Meso, Light Lidocaine, Lidocaine, Deep, Deep Lidocaine, Volume Lidocaine in manufacturer materials)
Approval / Certification
- CE marking reference: Neuramis Deep is shown as CE 1293 in the manufacturer product range table.
- Raw material approvals (scope clarification): According to Medytox Inc., the HA raw material has molecular weight approval referenced by EDQM and US FDA in their materials. This statement pertains to the HA material as described, and should not be interpreted as a universal regulatory approval of the finished product in every market.
Storage Information
- Storage temperature: 2°C to 25°C
- Storage conditions: cool, dry place (as listed)
- Shelf life: commonly listed as 24 months (from manufacturing date in competitor content)